Abstract
INTRODUCTION: Mantle cell lymphoma (MCL) is a distinct subtype of lymphoma and is characterized by an aggressive clinical course in the majority of cases. In older patients, commonly used induction regimens are either anthracycline-containing CHOP-like regimens or bendamustine, both in combination with rituximab, followed by rituximab maintenance [Dreyling, 2017]. Data on efficacy and safety of rituximab in MCL are mainly derived from the randomized GLSG2000 trial, showing higher remission rates and longer FFS with R-CHOP vs. CHOP, but no significant differences in PFS in remission and OS due to limited statistical power [Lenz, 2005]. We now performed a long-term ITT analysis of CHOP vs. R-CHOP from all GLSG2000 patients pooled with the CHOP patients of the preceding GLSG1996 trial.
METHODS: Of GLSG2000 and GLSG1996 trials, patients with advanced stage, previously untreated MCL, prospectively assigned to chemotherapy only (CHOP) or the combination with rituximab (R-CHOP), were included in this pooled analysis. Primary endpoints of this evaluation were FFS and OS, tested hierarchically. Secondary endpoints included PFS in first remission, OS after first treatment failure and rate of secondary malignancies. Exploratory subgroup analyses for FFS and OS were performed according to sex (female, male), MIPI (low, intermediate, high), Ki-67 (<30%, ≥ 30%) and cytology (non-blastoid, blastoid). Since only a subset of patients were randomized, statistical comparisons adjusting for MIPI score by Cox regression were considered as primary. For second line treatment, statistical comparisons adjusting for MIPI score and time to first treatment failure were done. A post-hoc calculation showed a statistical power of 80% and 90% to detect clinically relevant OS hazard ratios of 0.72 and 0.68, respectively.
RESULTS: Between 1996 and 2006, 385 MCL patients were included to receive either CHOP (n=201) or R-CHOP (n=184). Key patient characteristics were well balanced: Median age was 61 (37-86) versus 62 (35-84) years, 72 and 79% were male, median MIPI score was 5.78 (4.52-9.18, n=197) versus 5.80 (4.59-8.60 n=175), Ki67 ≥ 30% was seen in 17% (n=115) versus 17% (n=96) and blastoid morphology was seen in 8% (n=61) versus 8% (n=60) of patients treated with CHOP and R-CHOP, respectively. ASCT consolidation was done in 15% (n=31) and 21% (n=39) of CHOP and R-CHOP patients, respectively (p=0.15).
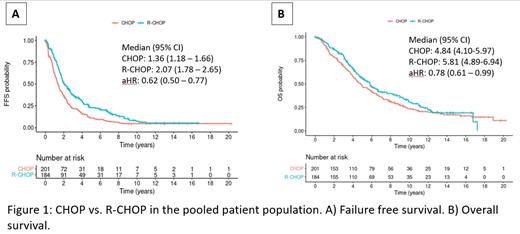
Median FFS was 1.36 (95% CI: 1.18 - 1.66) vs. 2.07 (1.78 - 2.65) years, adjusted HR 0.62 (0.50 - 0.77), p<0.0001. With a median follow-up of 13.4 years, median overall survival was 4.84 (4.10-5.97) vs. 5.81 (4.89-6.94) years with an aHR of 0.78 (0.61-0.99), p=0.039 (Figure 1 A-B). As for secondary endpoints, median PFS in remission was 1.48 (1.19 - 1.85) vs. 2.08 (1.65 - 2.65) years, aHR 0.67 (0.53 - 0.86), p=0.0012. Median OS after first treatment failure was virtually identical for both arms (2.67 (2.11 - 3.28) vs. 2.64 (2.06 - 3.44) years for CHOP vs. R-CHOP, aHR 1.09 (0.83 - 1.41, p=0.54). OS was however prolonged for patients receiving a rituximab-containing second-line treatment, with a median OS of 2.11 vs. 3.10 years (aHR 0.70 (0.54 - 0.91), p=0.0077) for non-rituximab vs. rituximab-based therapies. This effect was also present in the subgroup of patients pretreated with R-CHOP, confirming that rituximab maintains its benefits, even if repeated in relapse (aHR 0.55 (0.37 - 0.83)).
Regarding long term toxicity, the rate of secondary malignancies after 10 years was 0,5 and 3,9% for hematological and 7 and 8% for non-hematological malignancies for CHOP and R-CHOP, respectively.
In subgroup analyses, an efficacy of rituximab was seen across all MCL risk groups: HRs for FFS were 0.58 (95 % CI: 0.36 - 0.94) for MIPI high, 0.59 (0.41 - 0.84) for MIPI intermediate and 0.69 (0.49 - 0.97) for MIPI low (interaction p=0.80 for MIPI high vs. low; interaction p=0.50 for MIPI intermediate vs. low); aHRs for Ki67 ≥ 30% vs < 30% were 0.51 (0.25 - 1.05) vs. 0.73 (0.53 - 0.99; interaction p=0.056) and for blastoid vs. non-blastoid morphology 0.19 (0.04 - 1.03) vs. 0.77 (0.52 - 1.14; interaction p=0.19), respectively.
CONCLUSION: We present mature results of a pooled MCL cohort, now demonstrating a statistically significantly prolonged OS for the combined immuno-chemotherapy confirming the current standard of care in first line treatment of MCL.
Bittenbring: Gilead: Consultancy; Pfizer: Consultancy. Hübel: Gilead: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; Celgene: Consultancy; EUSA: Consultancy, Speakers Bureau. Schmidt: Novartis: Consultancy, Honoraria, Other: Travel support; Kite/Gilead: Consultancy, Honoraria, Other: Travel support, Research Funding; Takeda: Consultancy, Other: Travel support; BMS: Consultancy, Other: Travel support; Janssen: Other: Travel support. Glass: Kite: Consultancy; Roche: Consultancy, Research Funding, Speakers Bureau; Riemser: Research Funding; Novartis: Consultancy; Helios Klinik Berlin-Buch: Current Employment; BMS: Consultancy. Hüttmann: Gilead: Honoraria; Celgene: Honoraria; Lead Discovery Center GmbH: Consultancy; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Klapper: Takeda: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Regeneron: Consultancy, Research Funding; Amgen: Research Funding. Hiddemann: Janssen: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Unterhalt: Roche: Research Funding. Dreyling: Genmab: Consultancy; BeiGene: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; Amgen: Speakers Bureau; Astra Zeneca: Consultancy, Speakers Bureau; Abbvie: Research Funding; Novartis: Consultancy, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Gilead/Kite: Consultancy, Research Funding, Speakers Bureau; Bayer HealthCare Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal